For decades, the preferred method of airway management during a tonsillectomy was endotracheal tube (ETT) intubation. This process consists of a thin tube passed through the oral cavity and vocal chords to the trachea and requires insertion of a laryngoscope to visualize the patients airway. The introduction of the laryngeal mask airway provided physicians with a less invasive and safer mechanism for airway management. However, laryngeal mask airways are not compatible with commonly used oral gags, which keep the patients mouth open or tongue blades which keep the tongue down and out of the way during procedure.
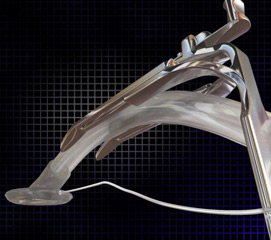
To solve this problem, Dr. Allan Allphin with Mercy-Clinic Ear, Nose and Throat, in cooperation with the Mercy Research & Development team, developed the Adjustable Tongue Blade (ATB), pictured.
The ATB is compatible with existing mouth gags, explains Keela Davis, director of Mercy Research & Development, but more importantly accommodates the laryngeal mask airway tube, allowing surgeons to use this newer technology to gain a secured airway for patients while making it much more user-friendly for the surgeon.
The patent-pending ATB was specifically designed to have variability in length, eliminating the need and cost associated with purchasing, cleaning and storing multiple sizes of tongue blades. The ATB is also compatible with existing mouth gags, making the transition to the new device seamless for surgeons and more comfortable for patients.
The Mercy R&D team prototyped the ATB concept which allowed me to better examine the design we had been working on. I was able to hold it, perfect it and offer additional changes to the design to ensure we are meeting the clinical need, Allphin explained.
By having functional prototypes available throughout the development of new products, practitioners are able to test the device in the clinical setting to confirm it does what it is intended to do. The ATB product is actively being marketed to surgical device manufacturers and medical device sales organizations for licensing of the novel and timely technology.
By developing this new adjustable tongue blade with a bigger channel to accommodate the laryngeal mask airway, we can reduce surgery time, lower costs associated with keeping multiple tongue blades on hand and create an overall better experience for the patient and surgeon, says Allphin.






Comments